By Daniel Collins | Jul 27, 2017 3:00 pm EDT
Spinraza revenue trends
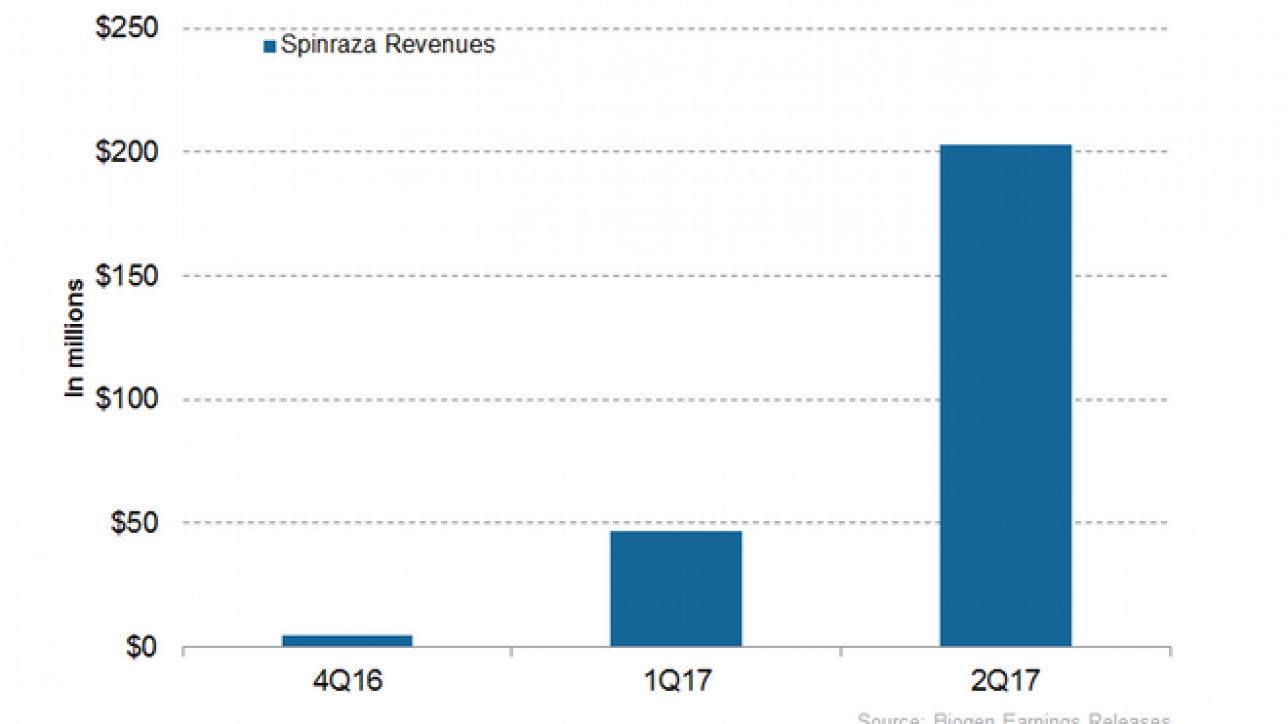
In 2Q17, Biogen’s (BIIB) Spinraza generated revenue of ~$203 million, compared to $47 million in 1Q17. In 2Q17, the US market generated revenue of ~$195 million for Spinraza, compared to $46 million in 1Q17.
Biogen is actively working with patients, payers, and physicians to expand Spinraza access to the highest possible number of spinal muscular dystrophy (or SMA) patients. Growth in the sales of Spinraza could boost the share price of the Health Care Select Sector SPDR ETF (XLV). Biogen makes up ~2.1% of XLV’s total portfolio holdings.
Recent approvals
In June 2017, the European Commission (or EC) granted marketing authorization to Spinraza for the treatment of 5q SMA (spinal muscular atrophy). 5q SMA is the most common form of SMA, and it accounts for ~95% of all SMA cases worldwide. The approval was granted based on the results of the Phase 3 ENDEAR trial and an interim analysis from the Phase 3 CHERISH trial.
In the Phase 3 ENDEAR trial, 51% of the patients who received Spinraza therapy achieved improvements in the definition of their motor milestone responders, compared to the 0% of patients that were on sham control.
In the Phase 3 CHERISH trial, children with late-onset SMA on Spinraza therapy demonstrated significant improvements in their motor functions compared to untreated patients. The Hammersmith Functional Motor Scale Expanded (or HFMSE) was used to measure improvements in motor function.
Patients treated with Spinraza demonstrated a difference of 4.9 points in the mean change from the baseline to month 15 in the HFMSE score compared to untreated patients.
Children who received Spinraza achieved 3.9 points of improvement at month 15 on the HFMSE scale, while untreated children depicted a mean decline of 1.0 point. The marketing approval of the drug in Europe is expected to further boost its revenue growth in 2017.
Biogen is also conducting a Phase 2 NURTURE trial to evaluate the effects of Spinraza in infants below six weeks old with genetically-diagnosed SMA and who were pre-symptomatic at the therapy’s initiation. Biogen’s peers in neurology market include Roche Holding (RHHBY), Pfizer (PFE), Merck & Co. (MRK), Novartis, and others. Notably, the Vanguard Health Care ETF (VHT) has ~1.7% of its total portfolio holdings in Biogen.